The interplay between physics and biology has led to significant advancements in our understanding of complex cellular behaviors, particularly in the realm of protein dynamics and compartmentalization. A recent study conducted by researchers at São Paulo State University (UNESP) sheds light on a fascinating phenomenon termed the Griffiths-like cellular phase, drawing parallels between concepts from condensed matter physics and biological structures. This revolutionary approach not only broadens our comprehension of cellular processes but also presents potential implications for disease management and the origins of life.
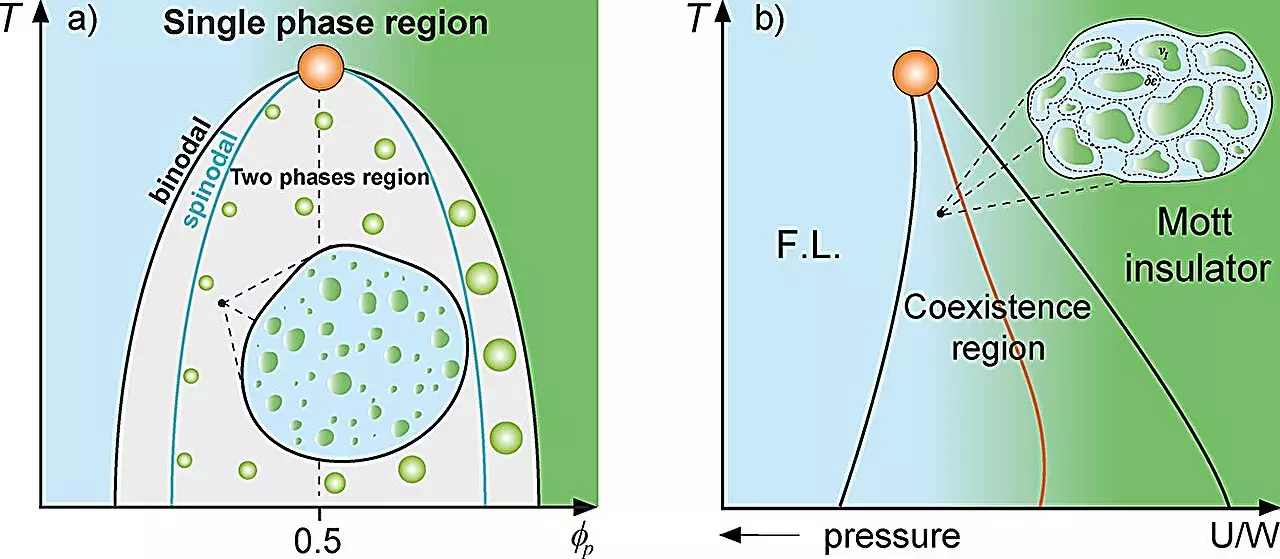
The research team, led by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, utilized classical mixture theory to model systems comprising two distinct substances, examining their interactions and phase coexistence. Classical mechanics has long been employed to explain the coexistence of various states of matter—most notably in scenarios such as supercooled water and the Mott metal-insulator transition. In their work, the researchers drew direct comparisons to these principles, examining how protein compartmentalization in cells mimics the behaviors seen in physical systems.
At the microscopic level, proteins can aggregate into droplets, resulting in a liquid-liquid phase separation pattern that can fundamentally alter their dynamics and functions. By identifying these phenomena as “rare regions,” the study emphasizes how certain spatial configurations of proteins can lead to significant behavioral changes, echoing the characteristics of the magnetic Griffiths phase in physics. This analogy serves as a profound reminder of how interconnected scientific fields can enable novel insights into fundamental biological processes.
The researchers employed thermodynamic frameworks—including the Grüneisen parameter, the Flory-Huggins model, and the Avramov-Casalini model—to analyze the dynamics of protein compartmentalization. Their findings suggest that near the binodal line determining phase separation, the dynamics of protein molecules slow considerably. Interestingly, this reduction in dynamics can also be observed at equivalent protein/solvent concentrations, proposing a novel Griffiths-like cellular phase.
This phase underscores the complexity of cellular environments and opens avenues for understanding how such conditions may contribute to key biological phenomena, including the origin of life. Particularly, the study connects its findings to Aleksandr Oparin’s theory from the 1930s, which posits that early life forms likely thrived in environments rich in coacervates—clusters of organic molecules within aqueous solutions. The slow dynamics of these early protein droplets may have played an integral role in their survival and evolution.
The study also delves into the concept of chirality, a property where certain molecules cannot be superimposed over their mirror images—a characteristic critical to the formation of biological molecules. The researchers propose that homochirality—the predominance of a single molecular chirality—was pivotal during the early stages of life, as it may facilitate orderly biological processes necessary for evolution. The coupling of increased protein diffusion times with reduced stochastic fluctuations in cellular environments could potentially optimize gene expression, a crucial aspect of life as we know it.
Beyond theoretical implications, the research by Souza’s team reveals significant prospects for understanding and addressing various diseases linked to protein dynamics and compartmentalization. Conditions such as cancer, neurodegenerative diseases, and even the effects of viral infections such as COVID-19 have been associated with the dynamics of protein droplets. By exploring how liquid-liquid phase separation affects disease pathology, the researchers highlight the potential for targeted therapeutic interventions.
For instance, the study posits that compartmentalization and the Griffiths-like cellular phase can impact gene mutation and tumorigenesis, providing a robust framework for future disease studies. Notably, the application of this research could influence how scientists approach therapeutic strategies targeting protein dynamics, potentially leading to innovative treatment methodologies for complex diseases.
The diverse contributions by co-authors from different institutions emphasize the significance of interdisciplinary collaboration in scientific advancement. The study exemplifies how merging concepts from physics, biology, and medicine can foster profound insights, paving the way for new discovery paths.
The research into Griffiths-like cellular phases not only enhances our understanding of cellular behavior but also offers promising implications for the origins of life and the treatment of diseases. By leveraging principles from diverse scientific domains, researchers are better equipped to unravel the complexity of life’s molecular mechanisms and their broader impacts on health and evolution. The findings mark a significant stride in our ongoing exploration of the intricate tapestry of life at the cellular level.
Leave a Reply