Lithium-metal batteries have long been hailed as the future of battery technology due to significantly higher energy densities compared to lithium-ion batteries. However, these lithium-metal cells have faced limitations such as short lifespans. Recent research by a team at the University of Science and Technology of China and other institutes has introduced a novel electrolyte design that could potentially overcome these limitations and pave the way for highly performing lithium-metal pouch cells with extended lifespans.
One of the primary challenges faced by current lithium-metal batteries is their limited cycle life of approximately 50 cycles, compared to around 1,000 cycles for commercial lithium-ion batteries. This lower lifespan can be attributed to issues such as the growth of lithium dendrites, the reactivity of lithium-metal, and high-voltage transition metal cathodes, which contribute to the constant degradation of the electrolyte. Despite extensive research efforts, the performance of lithium-metal batteries still falls short of expectations, with energy densities exceeding 500 Wh/kg and 1,000 cycles.
Approximately five years ago, researchers led by Prof. Shuhong Jiao developed an electrolyte that simultaneously stabilizes the anode-electrolyte and cathode-electrolyte interfaces in lithium-metal batteries, suppressing electrolyte degradation. This electrolyte design was based on in-depth studies of the physicochemical processes within lithium-metal batteries, highlighting the crucial role of electrolytes in dictating battery performance. The team leveraged affordable components and drew inspiration from prior research on novel classes of electrolytes to develop their groundbreaking design.
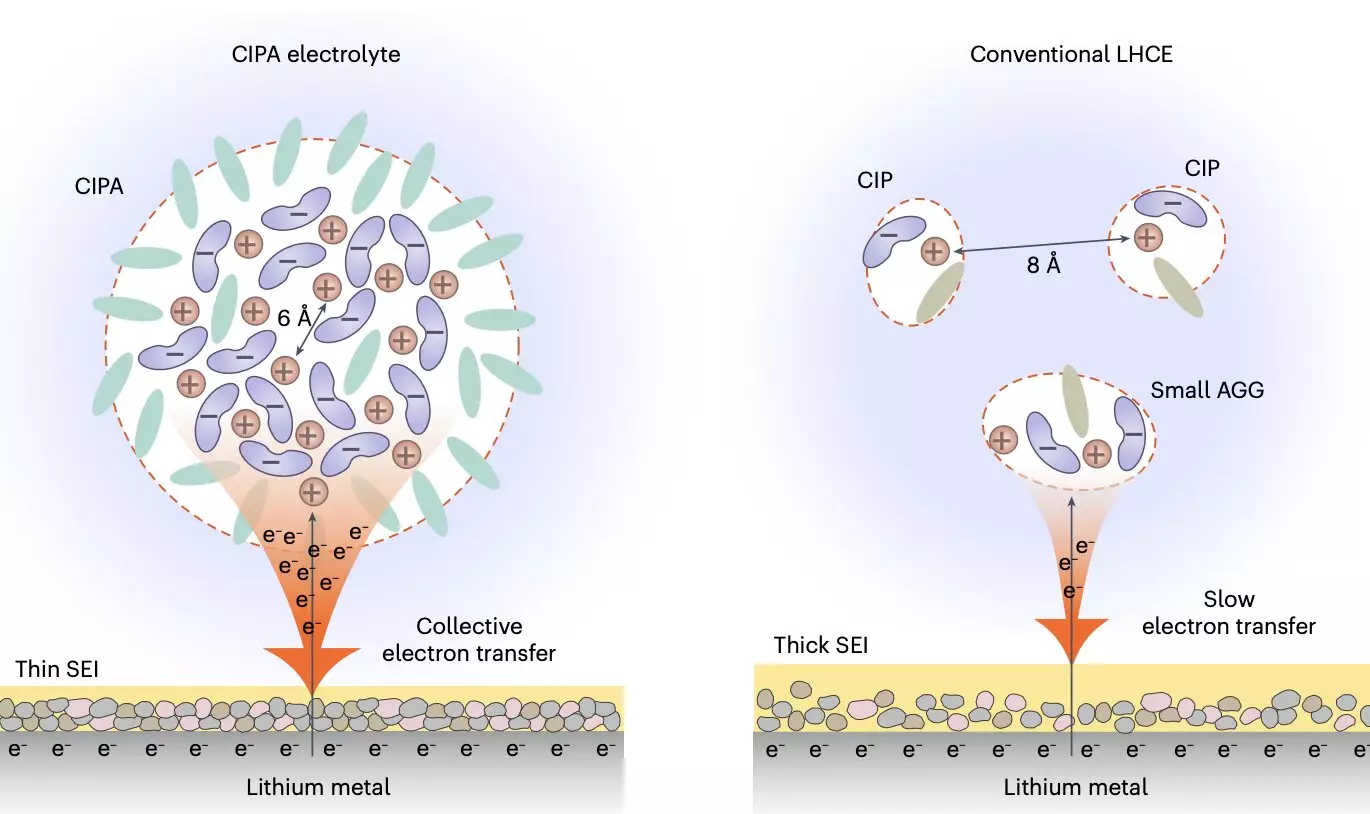
The recent study introduced a new class of electrolytes with a unique solvation structure, focusing on ion pairs that underlie the electrolyte’s aggregate formation. The electrolyte features large compact aggregates, known as compact ion-pair aggregates (CIPAs), formed by densely packed lithium-anion ion pairs with coordination bonding. This innovative design contrasts with conventional electrolytes, opening up new possibilities for enhancing battery performance and longevity.
The researchers’ electrolyte design exhibits collective reduction on the lithium-metal anode, promoting rapid reduction of anions in the CIPA structure and the formation of a thin, stable solid electrolyte interface (SEI). This process suppresses electrolyte decomposition, leading to homogeneous lithium deposition and reduced anode-specific areas, further mitigating degradation. Additionally, the electrolyte demonstrates good oxidative stability and inhibits transition metal dissolution from the cathode, improving the stability of both interfaces.
Initial tests with the newly designed electrolyte in a 500 Wh/kg lithium-metal pouch cell showed promising results, with 91% energy retention after 130 cycles. The researchers aim to extend the cycle life of these cells to over 1,000 cycles while exploring higher energy density targets of 600 Wh/kg with improved longevity. The mesoscopic solvation structure introduced by the team represents a significant advancement in electrolyte design for lithium-metal batteries, offering a new direction for future research and development in the field.
Leave a Reply