The quest for sustainable and efficient energy storage solutions has led researchers to explore alternatives to the widely-used lithium-ion batteries. Among the promising contenders is the sodium-ion battery (NIB), which employs sodium (Na) in place of lithium (Li). Sodium, an abundant element found in salt, offers a plethora of advantages over lithium, including accessibility and lower reactivity. This latter trait translates to improved electrochemical stability, making sodium-ion batteries particularly well-suited for rapid charging and operation in colder environments. However, the broader adoption of NIBs is hindered by some significant challenges associated with their performance and manufacturing processes.
Sodium-ion batteries face inherent limitations such as relatively lower energy density and a reduced lifespan compared to their lithium-ion counterparts. A key obstacle lies in the necessity of using hard carbon as the anode material. Unlike graphite, which is utilized in lithium-ion technology, hard carbon features larger interlayer spacing that accommodates the larger sodium ions. However, hard carbon does not naturally occur; it must be synthesized through a complex carbonization process. This involves heating hydrocarbon precursors—derived from plants or polymers—in an oxygen-free environment at temperatures exceeding 1,000°C. This intricate process is not only challenging but also presents significant economic and environmental hurdles that have stymied the scaling of sodium-ion battery production.
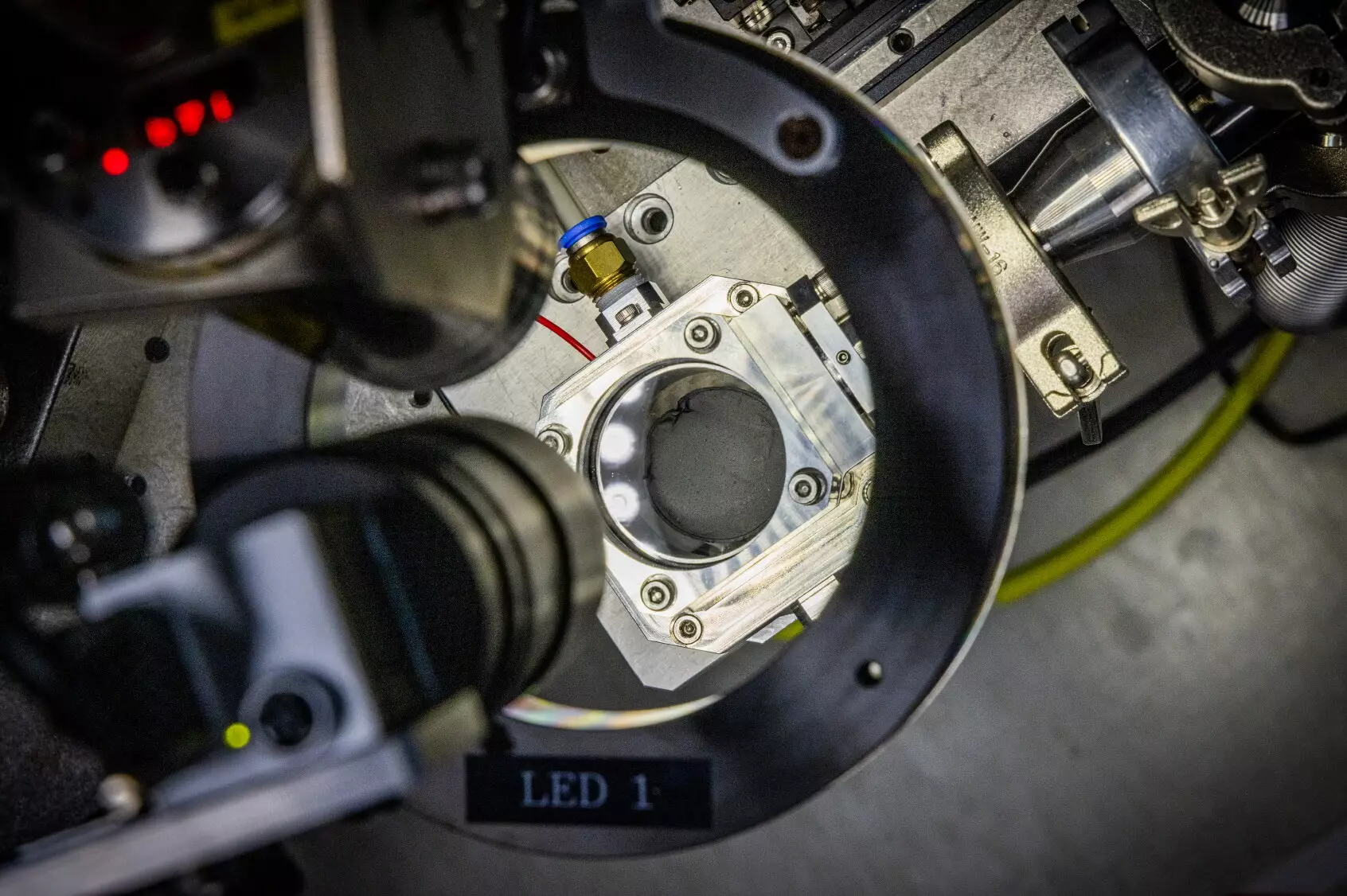
It is against this backdrop of challenges that a pioneering research team led by Dr. Daeho Kim and Dr. Jong Hwan Park has made a significant breakthrough. The team has developed a microwave induction heating technology that expedites the preparation of hard carbon anodes within a mere 30 seconds. By leveraging principles that are common in household microwave ovens, this innovative approach allows for rapid heating of the precursors to over 1,400°C, dramatically reducing the time and resources required for the carbonization process.
The team utilized a combination of polymer films and a minimal quantity of highly conductive carbon nanotubes. The microwave magnetic fields they employed facilitated localized heating, effectively addressing the manufacturing inefficiencies associated with conventional methods. This approach has garnered interest not just in the realm of sodium-ion batteries but across several industrial applications—most notably in the production of semiconductors and display technologies.
The success of this innovative heating technology can be attributed, in part, to the team’s “multiphysics simulation” methodology. By applying this advanced simulation technique, the researchers gained crucial insights into the dynamics involved when microwave electromagnetic fields interact with nanomaterials. This understanding was instrumental in creating an efficient process for synthesizing sodium-ion battery anode materials, ultimately leading to the publication of their findings in a reputable scientific journal.
The collaborative effort between academia and research institutions, embodied by the participation of student researchers Geongbeom Ryoo and Jiwon Shin, underscores the value of educational partnerships in driving technological advancements. This cooperative model has cultivated a fertile ground for groundbreaking research within KERI’s Nano Hybrid Technology Research Center, which is recognized as a leader in carbon nanomaterials technology in South Korea.
As interest in alternative battery technologies intensifies—particularly in light of growing safety concerns linked to electric vehicle fires—the relevance of sodium-ion batteries becomes increasingly pronounced. Dr. Park noted the advantages of their technology in addressing these safety issues while boasting performance benefits in low-temperature environments. The promise held by microwave induction heating extends beyond sodium-ion batteries; the team envisions its applicability in the development of all-solid-state batteries, which will require high-temperature sintering processes in their construction.
KERI is currently in the process of securing domestic patents, laying the groundwork for potential partnerships with industry leaders in energy storage. As manufacturers look to diversify their battery portfolios, this innovative technology promises to attract significant attention and may catalyze breakthroughs that will enhance the commercial viability of sodium-ion batteries.
The development of a time-efficient method for synthesizing hard carbon anodes could be a game changer for sodium-ion battery technology. As the team continues to refine their processes and evaluate new applications, the future of battery technology looks promising, with the potential for substantial advancements that could transform energy storage solutions globally.
Leave a Reply