The natural world and modern technology share a fascinating connection through the processes of energy conversion. Photosynthesis, which drives the life cycle of plants and bacteria, and the photovoltaic cells that convert sunlight into electricity, both hinge on the intricate dance of electrons within molecules. At the core of these phenomena is the movement and transfer of charges, which occurs at a molecular level with prompt implications for chemistry, physics, and engineering.
The Intricacies of Light-Induced Charge Transfer
When light is absorbed by a molecule, it prompts an immediate redistribution of electronic density—a phenomenon that occurs with remarkable speed, shaping our understanding of chemical reactions. This ultrafast process is not merely a static interplay of electrons but is intimately connected with quantum mechanics and molecular dynamics. Measurements that capture the electron and charge transfer dynamics with high temporal resolution enable scientists to delve deeper into the fundamental mechanisms of these processes. This research also holds the promise of guiding the engineering of chemical structures and properties to control or augment these fast-moving changes, potentially leading to improved technologies in energy capture and conversion.
Recent advances have showcased the effectiveness of surgical techniques that deploy ultrashort ultraviolet pulses, particularly those generated by high-order harmonic sources or free electron lasers. These tools enable researchers to probe the immediate responses of molecules during photoionization events, rendering insights into timescales from the femtosecond (10^-15 seconds) down to the attosecond (10^-18 seconds). Despite significant technological progress, the early stages of electron and charge transfer following photoionization remain enigmatic, necessitating further exploration.
A groundbreaking study published in *Nature Chemistry* by an international team of researchers from renowned institutions such as Politecnico di Milano, Madrid Institute for Advanced Studies in Nanoscience, and several universities in Spain, has promised to illuminate the obscured pathways of ultrafast molecular dynamics. Through the application of attosecond extreme-ultraviolet (XUV) pulses, the team has gleaned invaluable insights into the behavior of electron transfer in complex molecular systems, particularly donor-acceptor configurations.
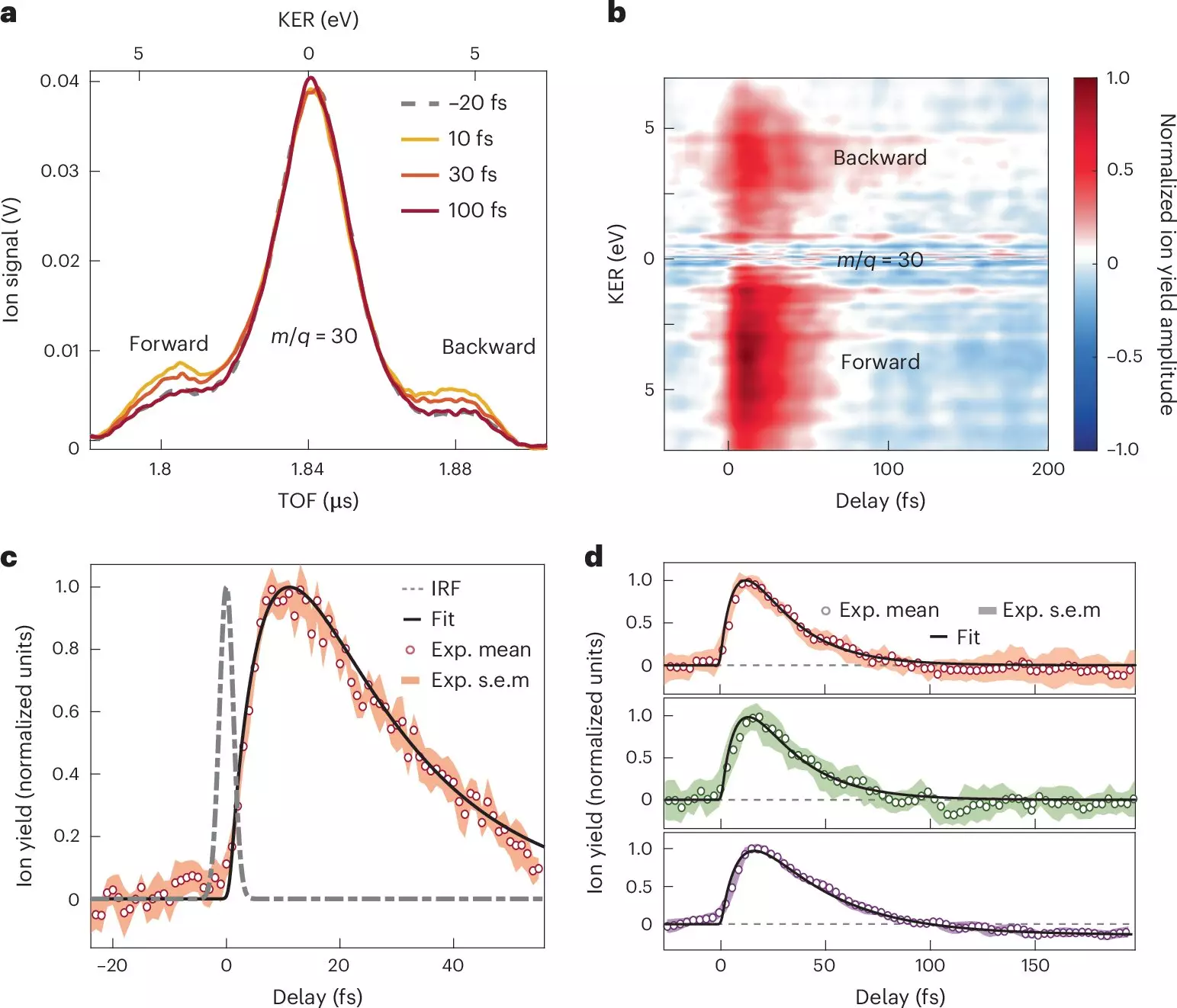
By examining nitroaniline molecules under these innovative conditions, the team successfully captured the initial phases of charge transfer with an unprecedented level of detail. Using an array of sophisticated techniques such as attosecond XUV-pump combined with few-femtosecond infrared-probe spectroscopy, alongside advanced many-body quantum chemistry computations, the researchers meticulously mapped the rapid sequence of events following photoionization. Notably, their findings demonstrate that electron transfer from the amino group, a donor unit, transpires in less than 10 femtoseconds, suggesting an incredibly swift interaction influenced by a synchronized movement of electrons and nuclei.
The study reveals significant findings, including a relaxation process that unfolds within 30 femtoseconds as nuclear wave packets extend across excited electronic configurations. These observations deepen our understanding of the electron-nuclear coupling phenomena that govern the behavior of electron donor-acceptor pairs, especially in contexts sensitive to light exposure.
One of the pivotal aspects of this work involves delineating the timeframes required for charge migration from the electron donor to the adjacent benzene ring within the molecular structure. This revelation not only provides critical information for academic inquiry but also challenges existing paradigms and pedagogical diagrams used to portray charge migration in organic chemistry.
The implications of such findings stretch beyond theoretical understanding; they signal potential advancements in practical applications associated with attosecond science. By refining our grasp of molecular dynamics at an unprecedented temporal scale, the work sets a compelling precedent for future investigations into the fundamental processes that underpin energy conversion technologies.
The revelations from this research signify an important evolution in our comprehension of molecular dynamics, especially in electron transfer phenomena. As scientists continue to leverage attosecond science, the prospects for developing advanced materials and technologies grounded in this fundamental knowledge appear boundless. The collaborative efforts showcased in the study not only enhance our theoretical frameworks but also promise to catalyze practical innovations in energy capture and use, paving the way for a cleaner and more efficient future.
Leave a Reply