Artificial photosynthesis stands at the frontier of sustainable energy solutions, merging the principles of natural photosynthesis with cutting-edge technology to tackle pressing environmental challenges. A remarkable advancement has emerged from the University of Michigan, where researchers have designed a system that excels in the conversion of carbon dioxide (CO2) into ethylene, a vital hydrocarbon primarily used in the production of plastics. This innovative method not only demonstrates impressive efficiency and yield but also holds the potential to significantly reduce atmospheric CO2 levels, aligning perfectly with global sustainability goals.
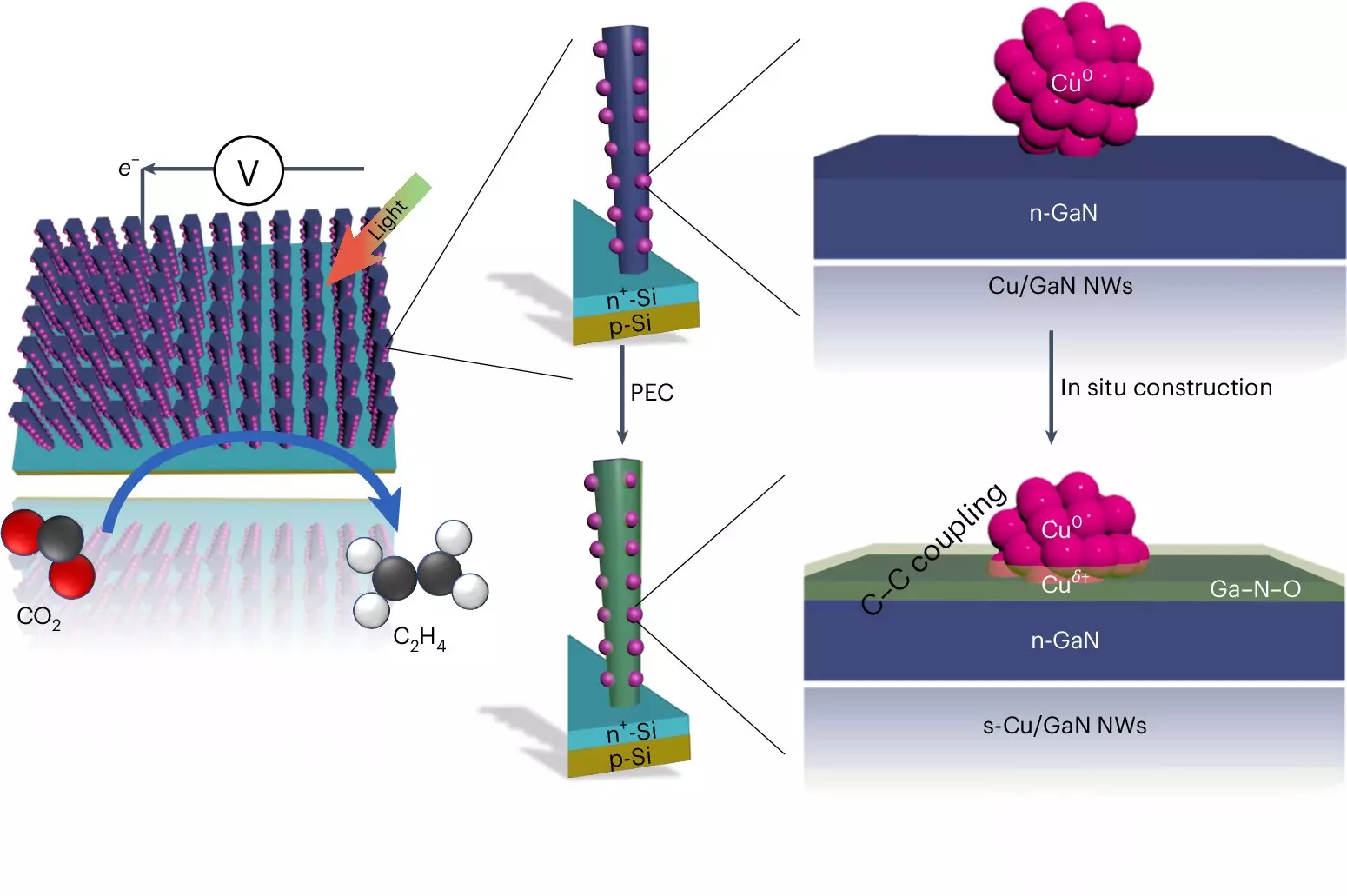
At the core of this artificial photosynthesis system is a sophisticated mechanism that chains carbon atoms while effectively managing the presence of oxygen. By utilizing two distinct types of semiconductors—gallium nitride (GaN) nanowires and a silicon base—the device captures sunlight and initiates a series of reactions. The GaN nanowires, minuscule structures measuring only 50 nanometers in width, facilitate the absorption of light that energizes electrons. This energy is then harnessed to split water molecules into hydrogen and oxygen. The freed hydrogen participates in the subsequent reaction with carbon dioxide, catalyzed by copper clusters embedded within the nanowires.
One of the standout features of this system is its extraordinary efficiency in converting CO2 into ethylene, outpacing other artificial photosynthesis methods by margins of five to six times. While conventional processes often rely on fossil fuels under extreme temperature and pressure, leading to CO2 emissions, this new approach offers a cleaner route by leveraging renewable energy. The researchers noted that a staggering 61% of the semiconductors’ free electrons contributed directly to the production of ethylene, demonstrating a powerful coupling between light absorption and chemical transformation.
The robustness of the system is noteworthy as well; it operated for 116 continuous hours without any decline in performance. This is particularly impressive given that alternatives, such as those employing silver and copper catalysts, exhibited significant degradation after only a few hours of use. The longevity of the Michigan team’s device can be attributed, in part, to the beneficial interplay between gallium nitride and the water-splitting process. The oxygen byproduct enhances the catalyst’s activity, fostering a self-healing mechanism that preserves its effectiveness over extended periods.
While the immediate output of this innovative system is ethylene, the researchers are setting their sights higher—literally. The overarching ambition is to synthesize longer chains of carbon and hydrogen to create liquid fuels, which would address the dual challenge of sustainable energy and carbon management. If successfully developed, liquid fuels could transform existing transportation technologies, making them more environmentally friendly and sustainable.
To achieve this goal, further refinements are necessary to optimize the reaction mechanisms for producing multi-carbon compounds, such as propanol, a three-carbon alcohol with significant utility in fuel applications. This is critical for expanding the potential impact of the artificial photosynthesis system.
The ramifications of this technology extend beyond ethylene production; they signify a pivotal shift in how industries could approach carbon management and sustainability. By capturing CO2 that would otherwise contribute to climate change and repurposing it for essential materials, the implications for reducing greenhouse gas emissions are profound. The transition from fossil fuel dependency to CO2 reuse could redefine the petrochemical industry, potentially leading to a circular economy model where carbon is not merely emitted but continuously recycled.
The artificial photosynthesis system forged at the University of Michigan epitomizes innovation at the intersection of chemistry and sustainability. With its superior performance in converting carbon dioxide into essential hydrocarbons, this technology holds promise not only for the creation of plastics but also for the broader aim of sustainable energy solutions. Continued research in this domain is paramount to unlocking the full potential of converting CO2 into valuable products, positioning it as a cornerstone of future environmental strategies. As scientists like Zetian Mi and Bingxing Zhang push the boundaries of what’s achievable, the prospect of a world where waste carbon is transformed into valuable resources inches closer to reality.
Leave a Reply